Conservators try to be as careful as possible when working with artifacts – objects left behind by people. They want to make sure they do not cause any damage as they clean and process each object. Each one should be treated a little differently depending on what it is made of. With this activity, we are looking at objects made of metal: precious or pure metal, like gold or silver.
Let’s begin with metals that are not precious, also referred to as poor. Metals like iron, when they are in the ocean’s salt water begin to rust, like a bicycle or outdoor chair left in the rain. This causes an encrustation or crusty surface to build around that piece of poor metal. Sometimes shellfish, like oysters, will attach themselves and add calcium carbonate from their glue that makes them stick on. This encrustation is called concretion and conservators use a special tool in order to remove the material from the object.
Here is a picture of a concretion and an x-ray to see what is in it.
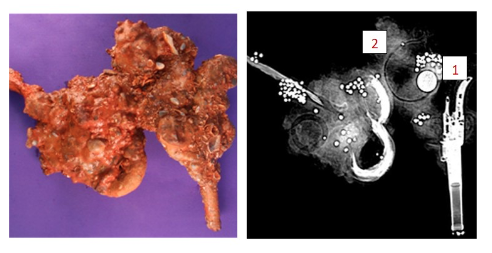
- Can you find the round, bright white artifact in the x-ray?1 This should be a very well preserved piece of metal, probably a pure metal, and could be a coin or a button. Do you see the “ghost” shadow ring just by it?2 That is more than likely a poor metal object that has deteriorated or slowly corroded away.
- Conservators may use vinegar to remove the calcium carbonate in a concretion that is on a soft metal, like a coin. The concretion can be removed without scratching the metal underneath.
Connect at Home:
- Put a raw egg into a ziplock bag and add enough white vinegar to cover the egg. A glass or a jar would work, too. For fun, add some food coloring (optional).
- Place into the refrigerator and let the egg sit in the vinegar for 24 hours. The egg doesn’t have to be in the refrigerator, but it needs to be away from sunlight.
- Remove from refrigerator and keeping the egg in the baggie, gently rub the outer shell to see if it disappears. If it doesn’t, then it may need to soak longer. If you used a jar or glass, remove the egg and gently rub under cold water to remove any shell remnants. This can also be done with the egg in a baggie.
- The vinegar removes the calcium in the shell, but the outer membrane remains.
- Either leave the egg in the baggie and pour out the vinegar or put the washed egg back in the baggie. While still in the bag the egg should feel rubbery, like a ball. Be careful— the membrane can easily break and when it does, you’ll see the egg yolk inside remains unharmed- like the coin! If the egg was in a jar or glass, grab a bowl or use your sink to gently bounce the egg, remembering the egg membrane can easily break.
- Throw away and wash your hands when done.
**Parents: It is the acetic acid in the vinegar that breaks down the calcium carbonate in the shell. The calcium floats away and the carbonate makes carbon dioxide, which were the bubbles you may have seen.

A second egg with food coloring and bubbles.

Pouring out the vinegar so can play with rubber egg in bag.

Some flashlight fun with the red egg.

From the top of the sink was when the egg popped. The egg is fine because of the protective membrane, which is in this photo. That’s how vinegar works to remove concretion on a coin without scratching or harming it!
Further your experiment! Try a hard boiled egg, try soaking for a longer period, try other liquids (soda, ammonia, etc.) and make sure to keep a record of your hypothesis and results!